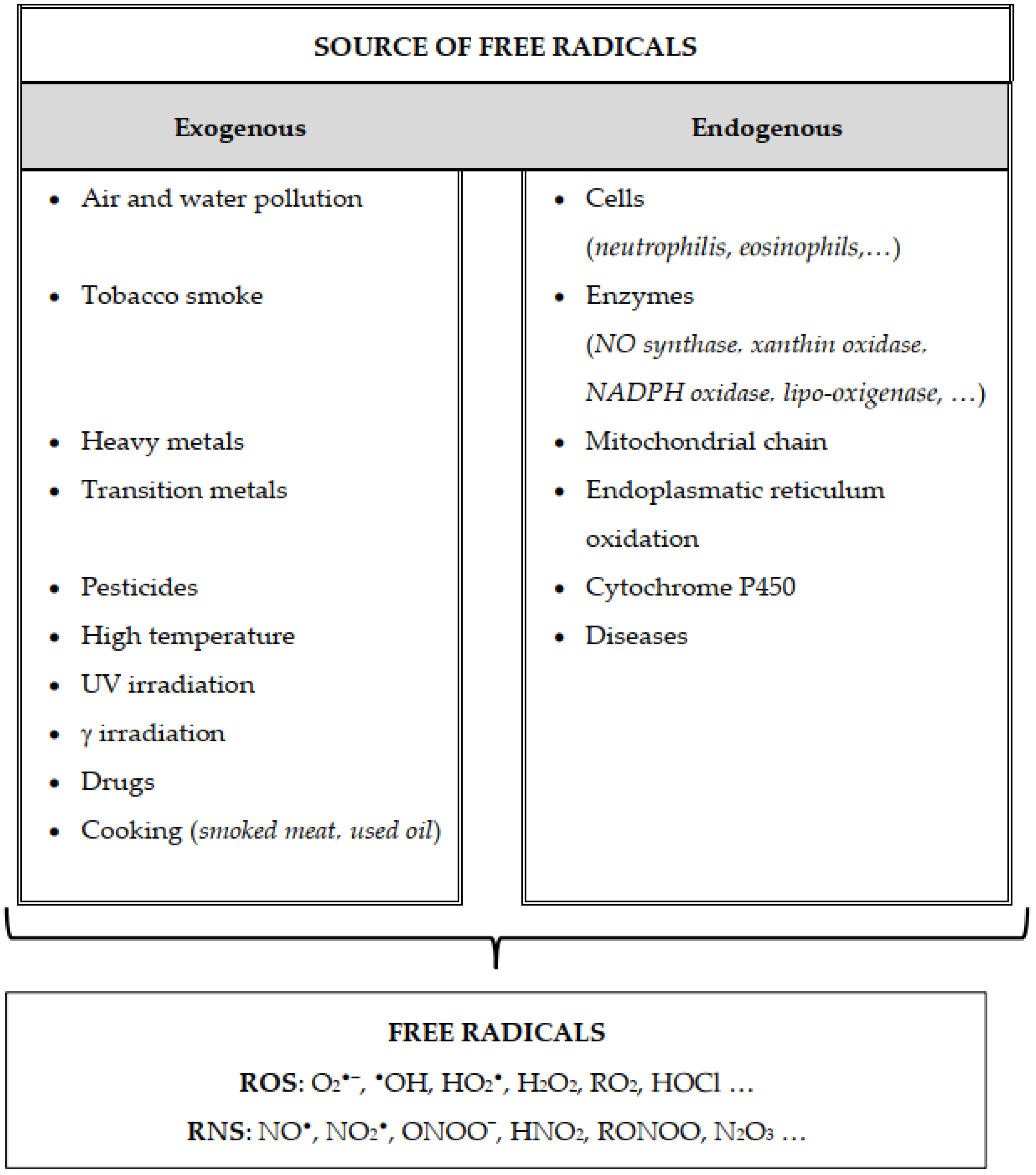
Solved overall reaction: 2NO(g)+O2(g)→2NO2(g) Step
5 (573) · € 15.50 · Auf Lager

For the reaction system 2NO(g) + O2(g)→ 2NO2(g) ; volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect
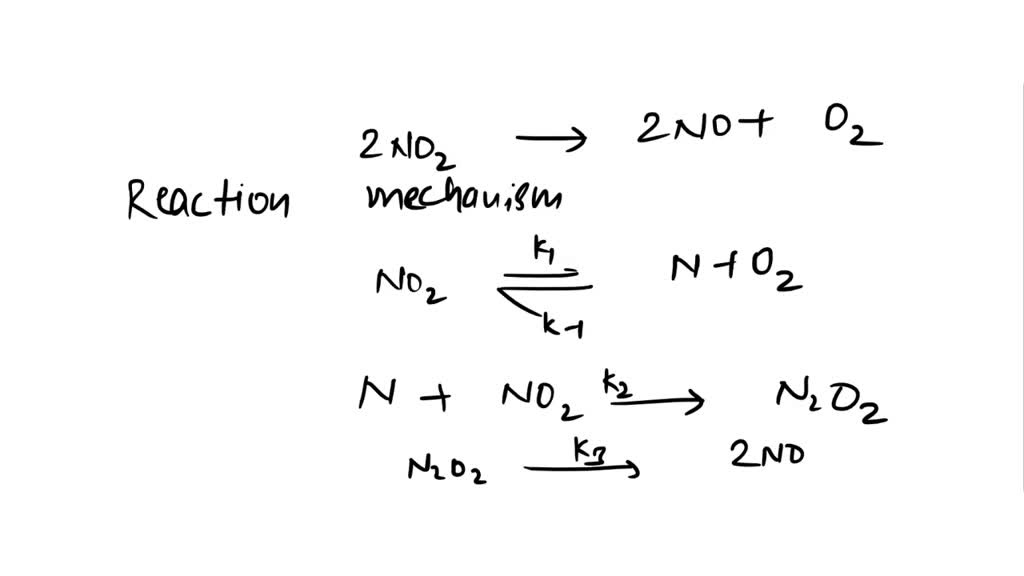
SOLVED: Given the overall reaction: 2NO2 (g) → 2NO (g) + O2 (g) and reaction mechanism: NO2 (g) → N (g) + O2 (g) N (g) + NO2 (g) → N2O2 (g)
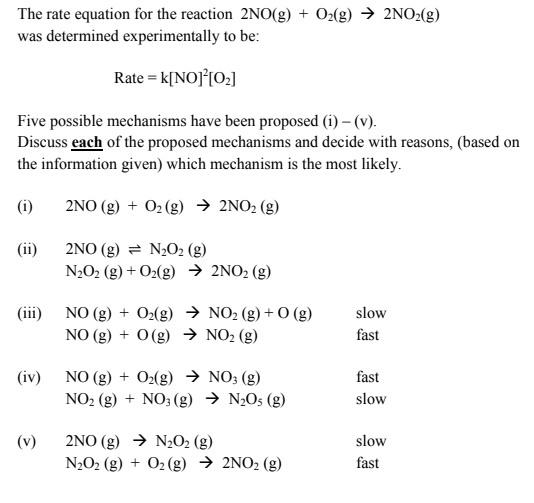
Solved The rate equation for the reaction 2NO(g) + O2(g) →
Following reaction takes place in one step: 2NO(g) + O2(g) ⇌ 2NO2(g) - Sarthaks eConnect

Solved 4) For the overall reaction, 2NO(g) + O2(g) →

Solved Question 17 The following two-step mechanism has been

Answered: 6.1 Calculate the enthalpy of the…

2NO(g)+O2(g)→2NO2(g) r=K[NO]2[O2

The reaction,2NO(g)+O(2)(g)hArr2NO(2)(g), is of first order. If the vo
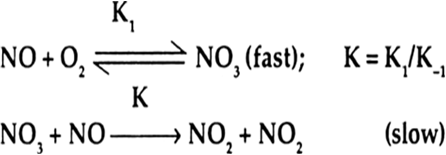
Nitric oxide, NO, reacts with oxygen to produce nitrogen dioxide

For a reaction, 2NO(g) + O2(g)→ 2NO2(g) Rate = k [ NO ]^2 [ O2 ] if the volume of the reaction vessel is doubled, then the rate of the reaction

Answered: 4.) The overall reaction 2NO2(g) +…
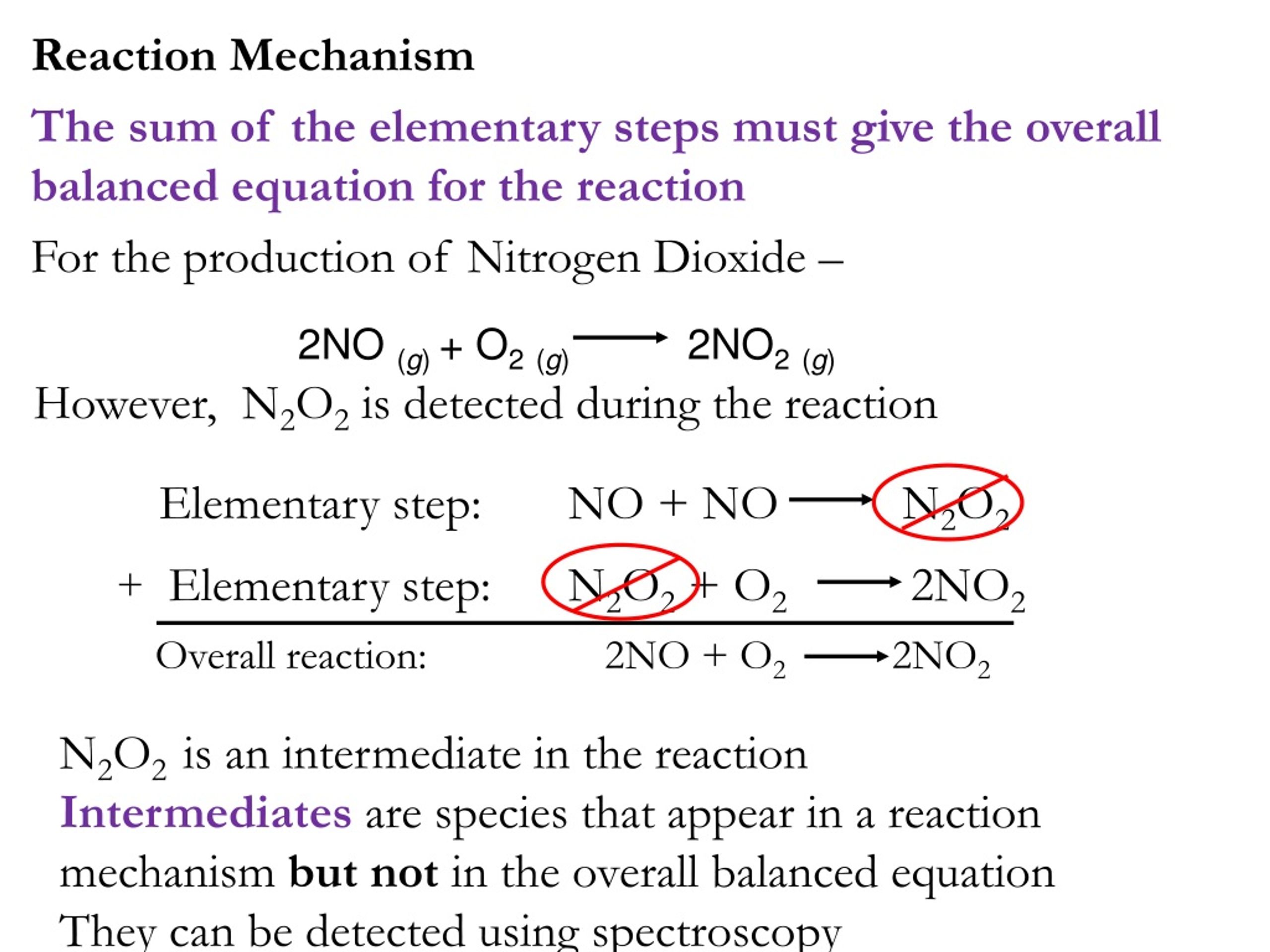
PPT - CHEMICAL KINETICS PowerPoint Presentation, free download - ID:1156612