- Startseite
- onoo 2
- Figure 2 from Understanding the fate of peroxynitrite in plant cells--from physiology to pathophysiology.
Figure 2 from Understanding the fate of peroxynitrite in plant cells--from physiology to pathophysiology.
4.8 (613) · € 21.50 · Auf Lager
Fig. 2. A hypothetical regulatory mechanisms of ONOO action in plant cells. In the biological milieu the peroxynitrite anion (ONOO ) is in equilibrium with peroxynitrous acid (ONOOH; pKa = 6.8). The reaction of ONOO with carbon dioxide leads to the formation of carbonate (CO 3 ) and nitrogen dioxide ( NO2) radicals. Alternatively, ONOOH can undergo homolytic fission to generate one-electron oxidants, hydroxyl OH and NO2 radicals. ONOOH readily crosses lipid bilayers and its decomposition to OH and NO2 radicals seems to become relevant in hydrophobic phases to initiate lipid peroxidation and lipid and protein nitration processes. If ONOO combines with SH-containing molecules (X-SH), it might be converted to S-nitroso compounds, e.g. S-nitrosoglutathione. Nitration and the formation of S-nitroso compounds are proposed mechanisms, by which ONOO regulates NO-dependent signaling events. On the other hand, nucleotides within DNA and RNA can undergo nitration by ONOO forming 8-nitroguanine, which may impart pathological consequences (Corpas et al., 2009b). - "Understanding the fate of peroxynitrite in plant cells--from physiology to pathophysiology."

Journal of Cellular Physiology, Cell Biology Journal

Mitochondrial Coenzyme Q Redox Homeostasis and Reactive Oxygen

Biological properties of mucus from land snails (Lissachatina

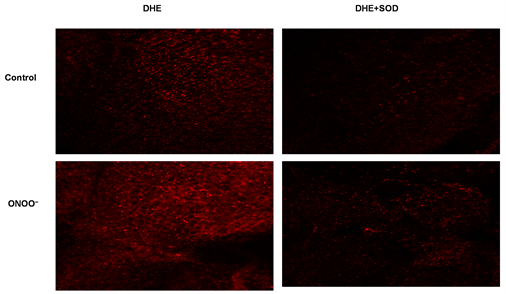
Overview of peroxynitrite reactivity with lipids. Å NO and

Journal of Cellular Physiology, Cell Biology Journal
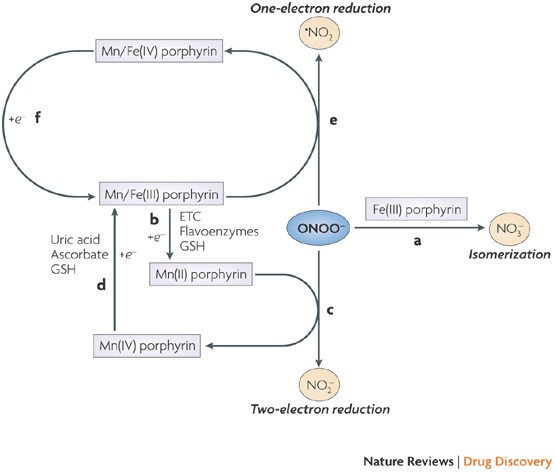
Peroxynitrite: biochemistry, pathophysiology and development of
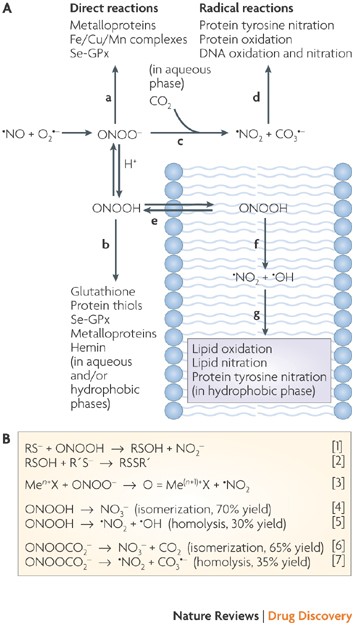
Peroxynitrite: biochemistry, pathophysiology and development of

ROS/RNS as molecular signatures of chronic liver diseases: Trends

Spontaneous Regression of Cancer: Revealing Granulocytes and

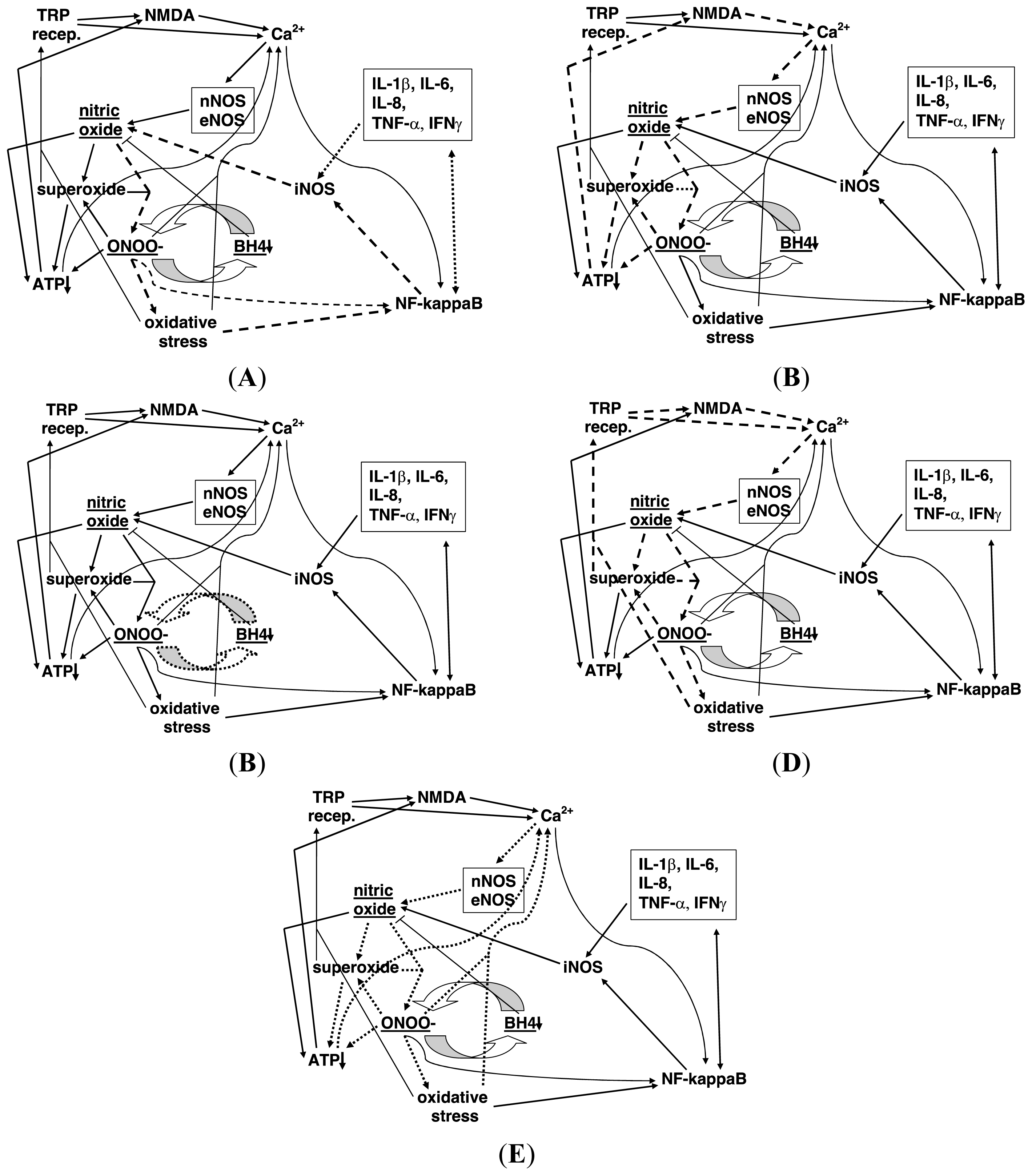
The roles of calcium and ATP in the physiology and pathology of
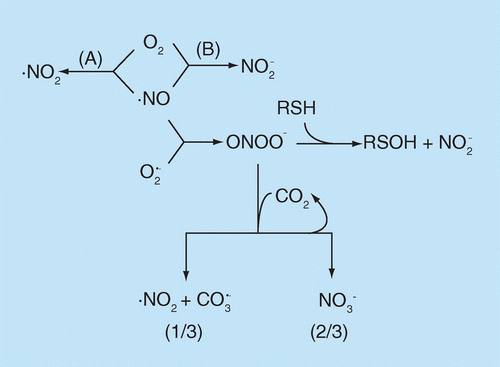
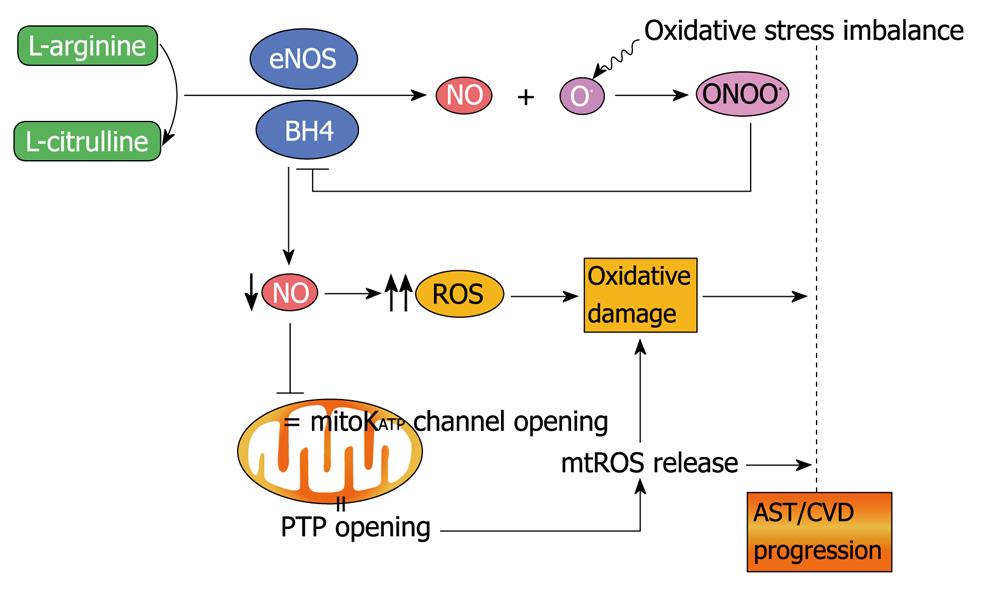
Nitric oxide: a brief overview of chemical and physical properties