- Startseite
- gloval eyes
- FDA: Global Pharma's eye drops contaminated with “filth” while made unapproved claims for eye drops
FDA: Global Pharma's eye drops contaminated with “filth” while made unapproved claims for eye drops
5 (242) · € 25.50 · Auf Lager

PDF) Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022
Witch's Pumpkin (Airdrop) - 🔥🔥 Check full Collection for other Amazing NFTs 🔥🔥 - NFTNAMA

An MBA. what's it worth? – SEGi College Sarawak

Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022

FDA Investigations Operations Manual - Public Health Information
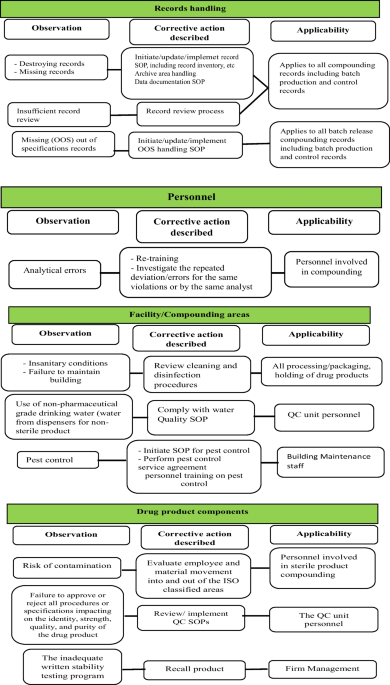
Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022

Robert Salcedo on LinkedIn: Unlocking the Potential of Cell and Gene Therapy: A Revolutionary Approach…

Content Analysis of US FDA Warning Letters Issued to Compounding Pharmacies Regarding Violations of Current Good Manufacturing Practices Between 2017 and 2022

Pharma Manager`s Association

USP Compounding Compendium 2018 - Flip eBook Pages 351-400

1 Introduction, Countering the Problem of Falsified and Substandard Drugs

PDF) HALVING THE PREMATURE DEATH RATE
Arthritis Supplements Reviewed by

Regulatory Affairs Professionals Society (RAPS) on LinkedIn: FDA: Global Pharma's eye drops contaminated with “filth” while made…